RESEARCH
Subunit exchange of hemoglobin α2β2 tetramer
- Subunit exchange between PEGylated and native Hbs
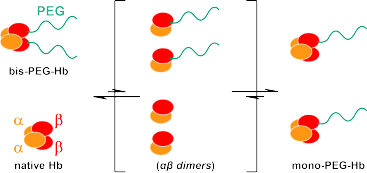
Fig. 1 Dimeric αβ subunit exchange between bis-PEGylated Hb (bis-PEG-Hb) and native Hb
through association-dissociation equilibrium of α2β2 tetramers.
Biomacromolecules 19(8), 3412-3420 (2018)
HDL:10564/3868 in institutional repository (the version post-peer review)
- Ring-opening polymerization via the association-dissociation equilibrium of Hb units
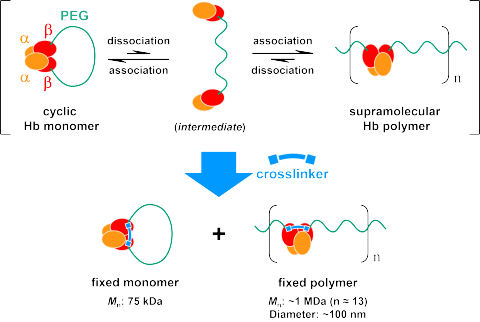
Fig. 2 Ring-opening polymerization of cyclic Hb monomer and subsequernt
crosslinking.
Biomacromolecules 20(4), 1592-1602 (2019) >> Journal Cover Image (Supplementary)
HDL:10564/3869 in institutional repository (the version post-peer review)
Title: Entropy-Driven Supramolecular Ring-Opening Polymerization of a Cyclic Hemoglobin Monomer for Constructing a Hemoglobin-PEG Alternating Polymer with Structural Regularity
Biomacromolecules 32(5), 1944-1954 (2021).
HDL:10564/3870 in institutional repository (the version post-peer review)
- Rheological properties of Hb-introduced hydrogels
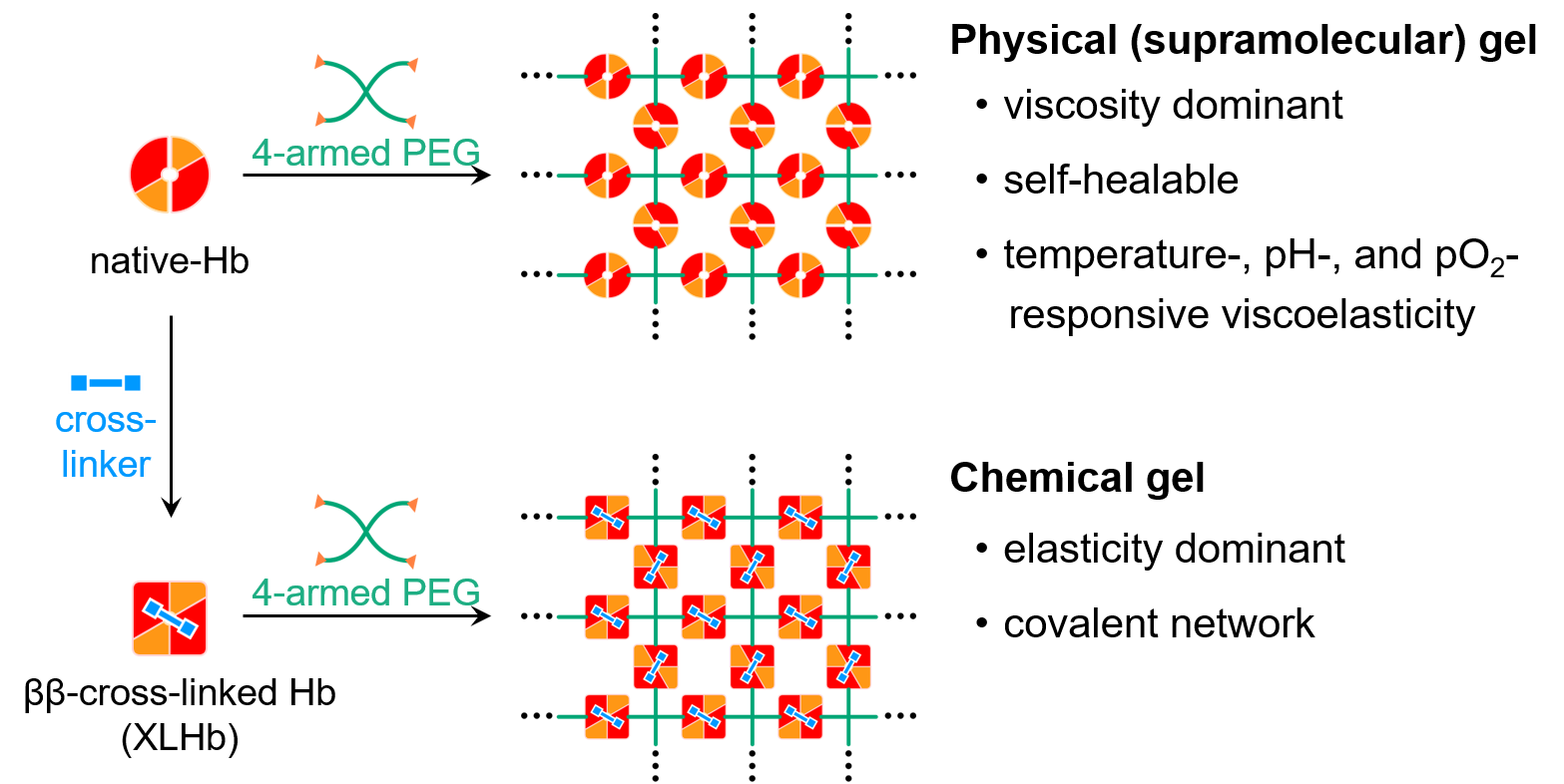
Fig. 3 Synthesis of Hb-introduced physical and chemical gels.
Biomacromolecules 26(8), 5212-5223 (2025). (open access)
Title: Rheological Properties of Hemoglobin-Based Physical and Chemical Gels, and Their Hybrid.
ACS Omega 11(1), 2194-2205 (2026). (open access)
Banners
SAKAI Laboratory
Postal Code 634-0813
Shijo-cho 88, Kashihara,
Nara, Japan